AAV Manufacturing Process from GenScript ProBio
Posted By GenScript ProBio
Body
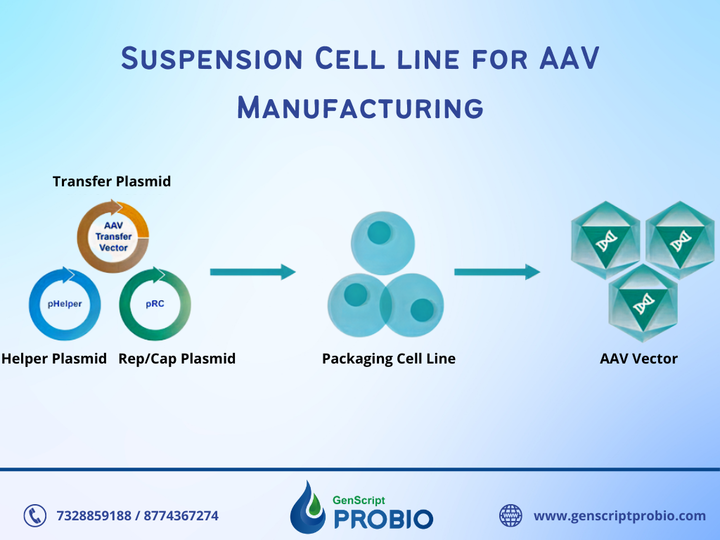 AAV manufacturing is appropriate for use in non-clinical phases. GenScript ProBio provides AAV manufacturing services suitable for use in non-clinical phases, such as animal studies on primates, toxicology studies, etc. We use a triple transient transfection system, with a manufacturing scale from 5L to 50L. We follow the phase-appropriate compliance to guarantee the products' safety in using non-clinical phases while reducing time and saving costs.
AAV manufacturing is appropriate for use in non-clinical phases. GenScript ProBio provides AAV manufacturing services suitable for use in non-clinical phases, such as animal studies on primates, toxicology studies, etc. We use a triple transient transfection system, with a manufacturing scale from 5L to 50L. We follow the phase-appropriate compliance to guarantee the products' safety in using non-clinical phases while reducing time and saving costs. 





Comments